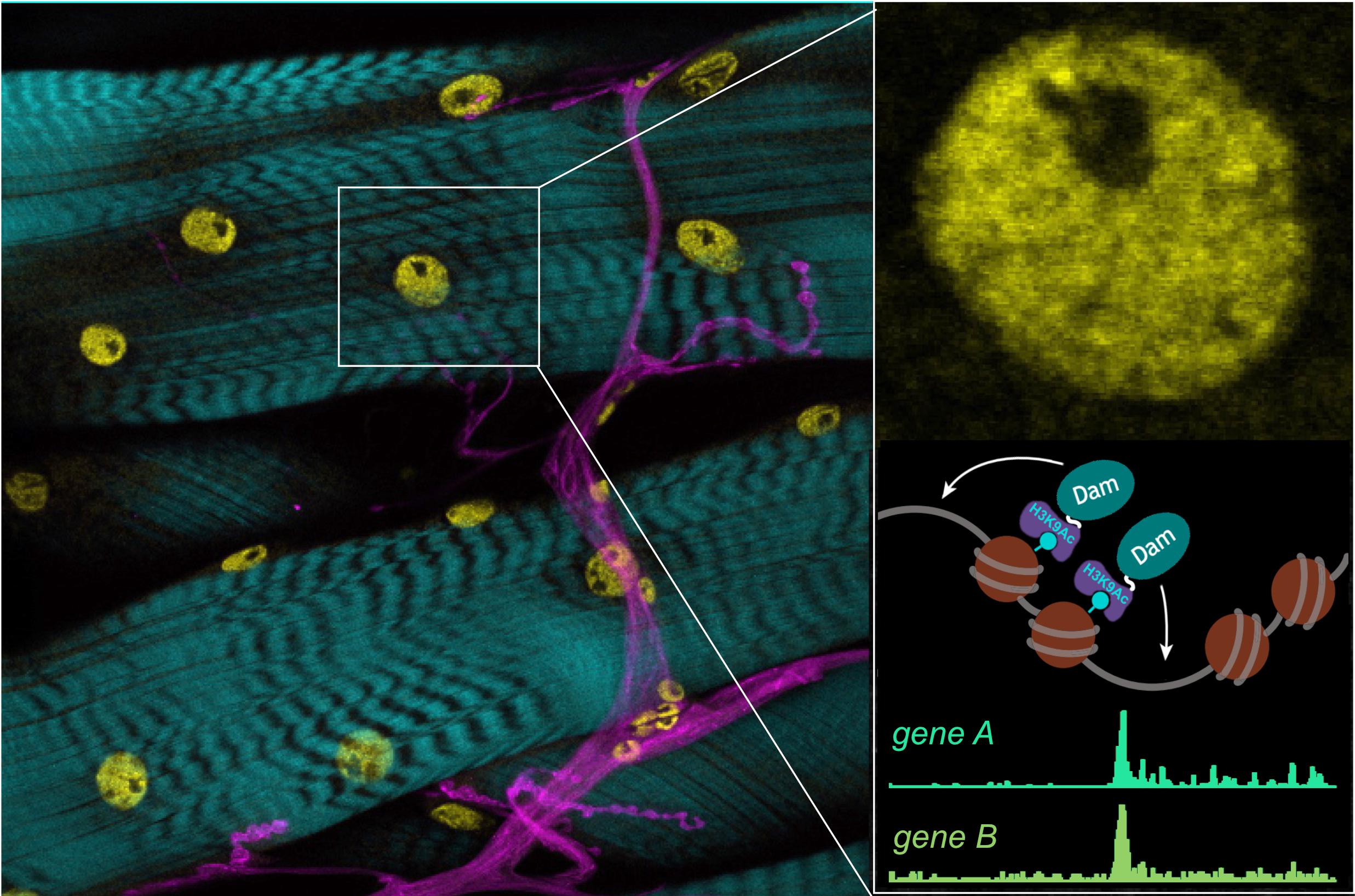
Image: Epigenetic marks in muscle nuclei (yellow), with nerves (magenta) and actin (cyan). Enlarged inset shows muscle nucleus and illustrates Chromatin-DamID strategy.
Epigenetic mechanism of critical period-plasticity in nervous system development
Supervisor: Professor Matthias Landgraf
Supervisor: Dr Jelle van den Ameele
Critical periods are important phases of brain development, during which plasticity is elevated to enable network tuning toward optimal, stable operation. The flip side is a concomitant vulnerability to disturbances, which can result in lasting maladjustment. In humans, forms of epilepsy and several psychiatric conditions are now thought to result from sub-optimal critical period experiences (e.g. during adolescence). The underlying mechanisms have largely remained elusive. Taking advantage of the relative simplicity of the fruit fly (Drosophila) as an experimental model, we recently identified the conserved mitochondrial-nuclear retrograde signalling pathway (mitochondrial reactive oxygen species activating Hypoxia-inducible factor-1alpha) as the primary signal for critical period plasticity.
Now we need to understand how a transient burst of this signal is converted into lasting change, e.g. of neuronal properties and therefore network output/behaviour. Using a targeted genetic screen, we have identified specific epigenetic modifiers that are required for long-term critical period-plasticity outcomes. The aim of this project is to investigate the processes that generate epigenetic signatures during critical periods of development. To this end, we have developed novel genetic tools for targeted Chromatin-DamID-seq, so we can study the role of these modifiers.
Type of work
In this project you will use novel genetic tools for cell type-specific Chromatin-DamID-seq to identify the changes in epigenetic marks that occur naturally during the critical period, and after perturbations. You will use a range of techniques to characterise how critical period-regulated changes in gene expression lead to changes in neuronal structure and function. These include advanced genetics, opto-genetics, functional imaging, electrophysiology and behavioural paradigms.
Importance of area of research concerned
Critical periods have been studied extensively in mammalian cortex, normally using system-wide manipulations. That makes clear interpretations of cause-effect relationships difficult. We have overcome this hurdle with the experimental system we have developed. It is unique in that it allows for cell-specific manipulations during the critical period. This offers a unique opportunity to discover and study mechanisms that are fundamental to nervous system development. It also provides us with the opportunity to investigate how we might re-open critical periods for therapeutic purposes, e.g. to allow poorly adjusted networks to correct themselves after experimental re-opening.
References
Sobrido-Cameán D, Oswald MCW, Bailey DMD, Mukherjee A, Landgraf M. (2023). Activity-regulated growth of motoneurons at the neuromuscular junction is mediated by NADPH oxidases. Front Cell Neurosci. 16:1106593. doi: 10.3389/fncel.2022.1106593.
Coulson B, Hunter I, Doran S, Parkin J, Landgraf M, Baines RA. (2022). Critical periods in Drosophila neural network development: Importance to network tuning and therapeutic potential. Front Physiol. 2022 Dec 2;13:1073307. doi: 10.3389/fphys.2022.1073307.
van den Ameele J, Trauner M, Hörmanseder E, Donovan APA, Battle OL, Cheetham SW, Krautz R, Yakob R, Gurdon JB and Brand AH (2024). Targeted DamID detects cell-type specific histone modifications in vivo. bioRxiv (pre-print); doi: https://doi.org/10.1101/2024.04.11.589050
