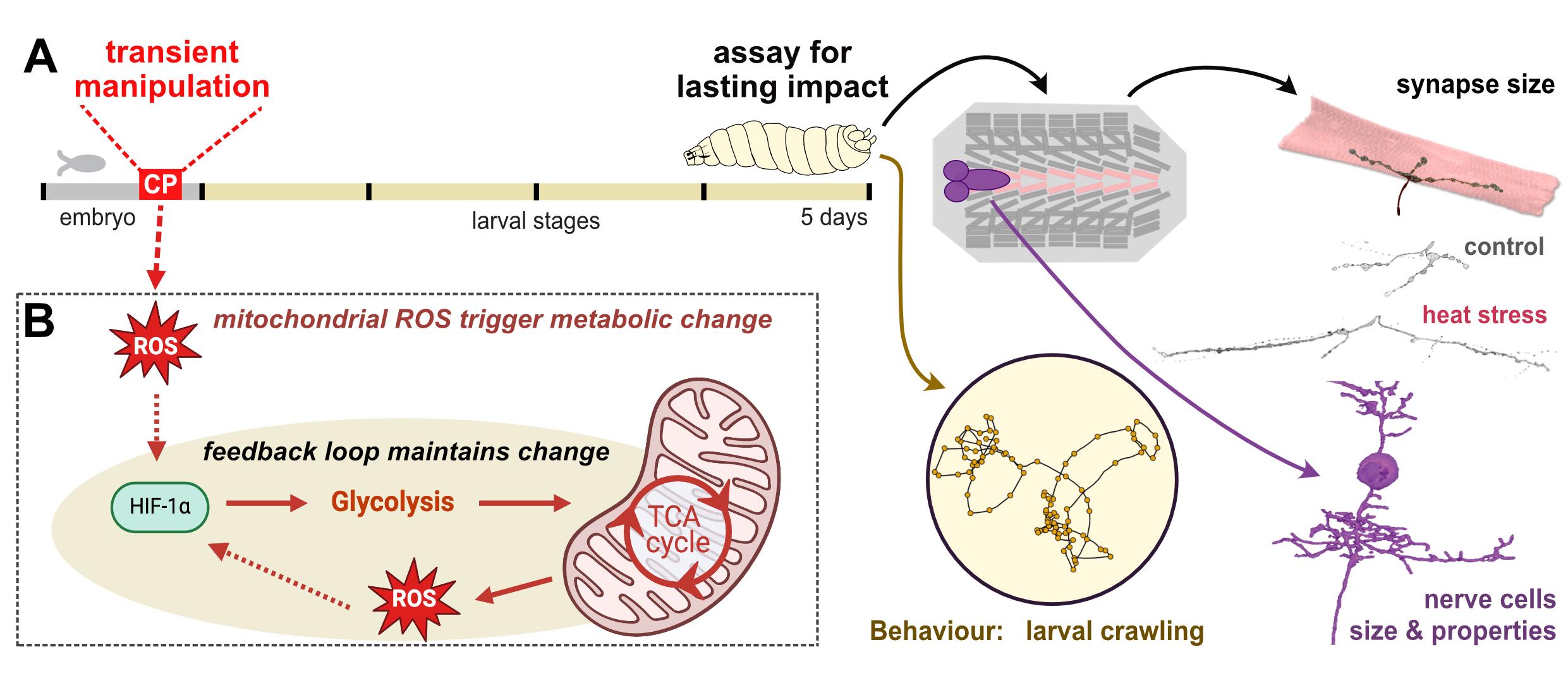
Image: Nerve cells assess their metabolic status during a defined critical period of development. Temperature challenges during this window can trigger metabolic feedback loops, which subsequently change neuronal growth and properties, and thus nervous system function and animal behaviour.
Metabolic regulation of brain development
Supervisor: Professor Matthias Landgraf
Temperature affects all biological processes. In cold-blooded animals, such as insects, developmental temperature dictates how their nervous system develops (e.g. numbers and types of connections formed), with consequences to function, stability and behaviour. For example, transient heat stress during a few hours of embryonic development of Drosophila embryos, creates animals, with unstable nervous systems that are prone to seizures and reduced locomotion.
We discovered that during critical periods of nervous system development, temperature changes mitochondrial metabolism. This triggers metabolic signals, which cause lasting changes, akin to metabolic re-programming seen in cancer cells.
We now need to understand how cellular metabolic need during normal development directs developmental decisions. We have developed a uniquely tractable experimental system. This has already led to the discovery of highly conserved, fundamental signalling pathways.
Type of work
This project will use a range of techniques, including opto- and thermo-genetics for targeted manipulations, as well as pharmacological manipulations; RNA seq and metabolomics, confocal imaging and behavioural assays to study the consequences of critical period manipulations.
Importance of the area of research concerned
How nerve cells acquire their properties during development, especially during so-called critical periods, is not well understood. This project takes a novel, metabolic perspective, which could radically change our understanding of the underlying mechanisms.
This knowledge should contribute toward developing therapeutic approaches that might enable re-programming neuronal properties, e.g. to ameliorate hyperexcitability of cells that causes epilepsy.
References
Drummond-Barbosa D, Tennessen JM (2020). Reclaiming Warburg: using developmental biology to gain insight into human metabolic diseases. Development. 147(11):dev189340. doi: 10.1242/dev.189340.
Coulson B, Hunter I, Doran S, Parkin J, Landgraf M, Baines RA. (2022). Critical periods in Drosophila neural network development: Importance to network tuning and therapeutic potential. Front Physiol. 2022 Dec 2;13:1073307. doi: 10.3389/fphys.2022.1073307.
Hunter I, Coulson B, Pettini T, Davies JJ, Parkin J, Landgraf M, Baines RA. (2024). Balance of activity during a critical period tunes a developing network. Elife. 12:RP91599. doi: 10.7554/eLife.91599.
Kiral FR, Dutta SB, Linneweber GA, Hilgert S, Poppa C, Duch C, von Kleist M, Hassan BA, Hiesinger PR. (2021). Brain connectivity inversely scales with developmental temperature in Drosophila. Cell Rep. 37:110145. doi: 10.1016/j.celrep.2021.110145.
