Research
Traditionally we believe that the ability to perform complex behaviour arises as the size of the brain or neural network increases. However, animals with anatomically small networks, like the nematode C. elegans, are capable of complex behaviours such as learning and maze navigation. This raises the question; how do these small networks generate behavioural complexity and are these mechanisms conserved through evolution?
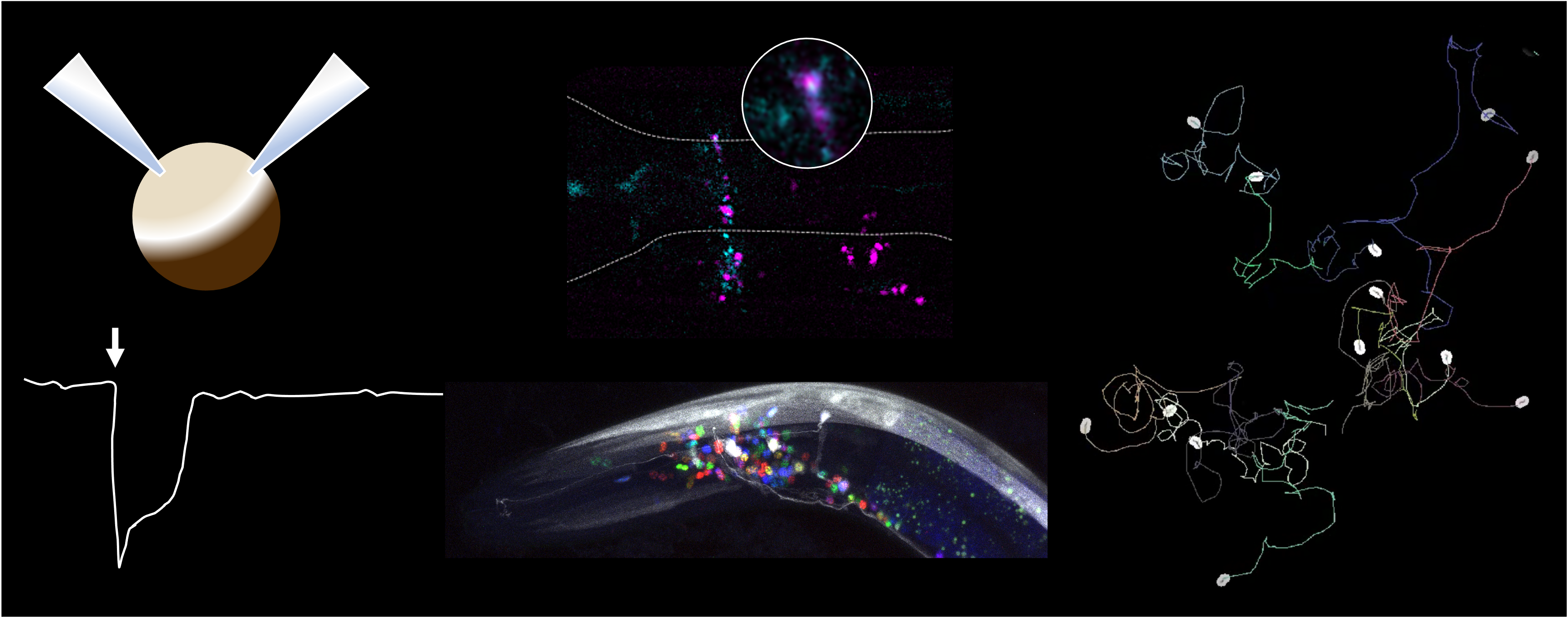
Our previous work uncovered the surprising extent of molecular complexity within the C. elegans nervous systems. this paired with its completely mapped connectome, makes it the ideal model to study this question. Specifically, the discovery of both excitatory and inhibitory fast-acting ligand gated ion channel (LGICs) for the same neurotransmitters, expressed within a single neuron, open avenues for new, yet to be discovered, mechanisms for behavioural plasticity and circuit remodelling during learning.
Our lab focuses on understanding how the nervous system processes these opposing signals to generate complex behaviour. To do this we have three main research areas:
Generating a high-resolution map of molecular complexity:
We are using high resolution imaging to resolve the synaptic localisation of neurotransmitter receptors and plan to use this data to build a functionality relevant connectome.
How does this molecular complexity affect behaviour:
Using high throughput tracking and behavioural assays, we are studying how changes in the localisation and function of neurotransmitter receptors influences circuits during learning.
Is there more complexity to be discovered:
We are continuing to delve deeper into the molecular complexity of C. elegans, by studying the functional importance of novel neurotransmitters and LGICs. Currently we are investigating how the polymodal channel, LGC-39, which is capable of binding chemically diverse neurotransmitters in vitro, functions in vivo. In the long term we aim to discover new types of LGICs which may yet change our understanding of this fundamental group of proteins and provide a wealth of future research opportunities.
Recent Publications
Hardege, I. et al. A Novel and Functionally Diverse Class of Acetylcholine-gated Ion Channels. Journal of Neuroscience (2023). https://doi.org/10.1523/JNEUROSCI.1516-22.2022
Morud, J., Hardege, I. et al. Deorphanization of novel biogenic amine- gated ion channels identifies a new serotonin receptor for learning. Curr. Biol. (2021). https://doi.org/10.1016/j.cub.2021.07.036
Hardege, I. et al. Neuronally Produced Betaine Acts via a Ligand Gated Ion Channel to Control Behavioural States. PNAS (2022). https://doi.org/10.1073/pnas.2201783119
