Methods and Techniques
Methods we use |
For quantitative measurements and analysis of behaviour we have a trackball system to monitor walking in insects, we employ optoelectronic cameras to record micrometer movements of insect appendages and we use high speed video cameras to capture episodic sequences of motor activity.
Recording neuronal activity: Motor activity is monitored with EMG recordings, and activity of sensory or motor axons with extracellular hook or suction electrodes. Single or paired intracellular sharp microelectrode recordings are used to reveal synaptic and spike activity of single neurons and to apply dyes and tracers for structural imaging.
We use calcium imaging to reveal the spatial dimension of neural processing in single neurons and networks. Calcium reporters are either injected intracellular via microelectrodes or by electrophoretic application through the neuronal sheath with a suction electrode.
Systemic and local application of neuroactive agents is used to modify behaviour and to reveal its neurochemical basis. Singing behaviour in crickets and grasshoppers can reliably released by injection of cholinergic agonists into the brain.
We use a laservibrometer/interferometer to measure submicron oscillations in hearing organs or to record the walking and vibration pattern of courting Drosophila.
Finally these measurements are supported by a quantitative data analysis using our custom made and tailored software tool NEUROLAB for the processing of behavioural and neurobiological data.
The range of genetic techniques in crickets is still very limited, but this may change within the near future. Combining the option of genetically calcium reporters with the ease of intracellular recordings will make the cricket an outstanding model system in neuroscience.
Some Innovations |
I have always been interested to develop new methods for our behavioral and neurophysiological experiments. A few of these are:
- using Peltierelements to reversibly block the activity of auditory organs in crickets and bushcrickets
- a device for changing the air pressure in insect tracheal systems
- light guide and laser technology to measure the wing movements of flying locusts
- electrophoretic delivery of calcium reporters into insect nervous systems
- new design of micro-electrode holders and suction electrodes
- a fast trackball system to measure insect walking and cricket phonotaxis
- software for the analysis of neurobiological data
- a catadioptric device to measure movements of insect appendages
- a servo-motor system to dynamically deliver sound stimuli from precisely defined different directions
Any interest please let me know.
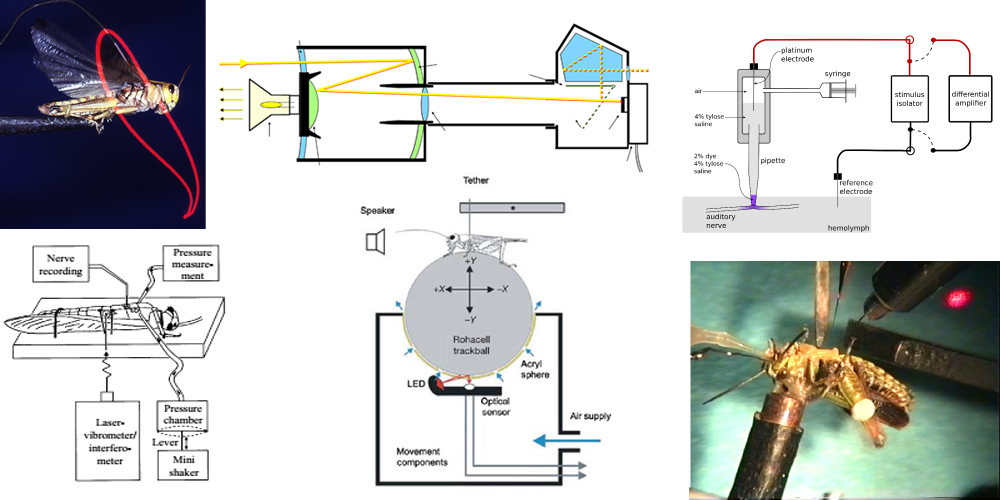
Wing movements of a locust, an optoelectronic camera, electrophorectic injection of tracers, injecting air into the locust tracheal system, a trackball for walking and brain stimulation in grasshoppers.
References:
- Isaacson MD and Hedwig B. (2017) Neuroanatomical and functional labeling by tracer electrophoresis through the nerve sheath. Scientific Reports, 7:40433, DOI: 10.1038/srep40433
- Hedwig, B and JFA Poulet (2005) Mechanisms underlying phonotactic steering in the cricket Gryllus bimaculatus (de Geer) revealed with a fast trackball system. J Exp Biol 208: 915-927
- Hedwig B (2000) A highly sensitive opto-electronic system for the measurement of movements. J Neurosci Meth, 100:165-171
- Hedwig B, Becher G (1998) Forewing movements and intracellular motoneuron stimulation in tethered flying locusts. J Exp Biol, 201:731-744
- Meyer J, Hedwig B, (1995) The influence of tracheal pressure changes on the responses of the tympanal membrane and auditory receptors in the locust Locusta migratoria L. Laservibrometric, laserinterferometric and neurophysiological studies. J Exp Biol, 198:1327-1339
