Critical periods of nervous system development - attaining stable function in the face of unpredictable biological and environmental availability
How can stable function emerge from developing brains in the face of unpredictable variability? Variability is inherent in all biological systems and accentuated by environmental changes, notably in (seasonal) temperature.
Key to this process is the developmental transition between network construction and the emergence of network function. These transitions are tuning phases, also called ‘critical periods’. They calibrate neuronal properties and thereby define network function, stability and capacity for future plasticity.
We find that critical periods are the developmental window when homeostatic setpoints - all important for network stability - are specified. This explains why transient disturbances during a critical period create lasting errors. In humans, many neuro-developmental conditions, including epilepsy, are thought to be the result of suboptimal critical period experiences (e.g. in adolescence).
We study the underlying mechanisms, using state-of-the-art genetics to manipulate single nerve cells precisely, and super-resolution microscopy to visualise connections between nerve cells. Electrophysiology reveals changes in excitable properties and cell-cell communication, while behavioural analysis gives a systems-level readout. RNA Seq and mapping epigenetic modifications identifies molecular mechanisms.
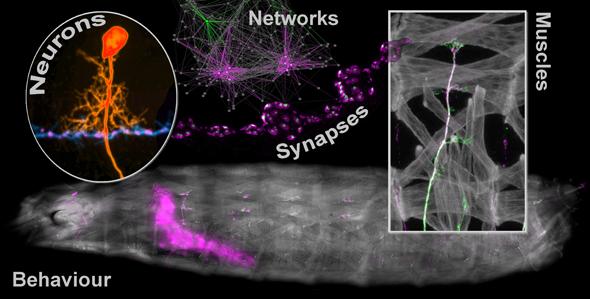
As an experimental model we work with Drosophila, because these questions are fundamental to all nervous systems and the mechanisms are conserved. The Drosophila model system is experimentally highly tractable, allowing manipulation of any cell at any time, an unprecedented level of experimental access to study critical periods.
So what are the signals that instruct network tuning? We have identified metabolic reactive oxygen species as candidates and think that metabolic activity might be the basic information currency. We are now investigating how transient developmental experiences lead to lasting changes in gene expression and cellular properties.
- A model synapse to study mechanisms of critical period adjustments
- Calcium dependent mechanisms to establish neuronal homeostatic setpoints
- Signals of change and mechanisms of maintenance
- Network connectivity is shaped by critical periods of development
- Computational modelling approaches to study the emergence of network function
Key Publications
Giachello CNG, Fan YN, Landgraf M, Baines RA. (2021). Nitric oxide mediates activity-dependent change to synaptic excitation during a critical period in Drosophila. Sci Rep. 11:20286. DOI: 10.1038/s41598-021-99868-8.
Dhawan S, Myers P, Bailey DMD, Ostrovsky AD, Evers JF, Landgraf M. (2021). Reactive Oxygen Species Mediate Activity-Regulated Dendritic Plasticity Through NADPH Oxidase and Aquaporin Regulation. Front Cell Neurosci. 2021; 15: 641802. DOI: 10.3389/fncel.2021.641802.
Valdes-Aleman J, Fetter RD, Sales EC, Heckman EL, Venkatasubramanian L, Doe CQ, Landgraf M, Cardona A, Zlatic M. (2021). Comparative Connectomics Reveals How Partner Identity, Location, and Activity Specify Synaptic Connectivity in Drosophila. Neuron. 2021 Jan 6; 109(1): 105–122.e7. DOI 10.1016/j.neuron.2020.10.004.
Oswald MCW, Brooks PS, Zwart MF, Mukherjee A, West RJH, Giachello, CNGMorarach K, Baines RA, Sweeney ST and Landgraf M. (2018). Reactive Oxygen Species Regulate Activity-Dependent Neuronal Structural Plasticity. eLife, 7. DOI: 10.7554/eLife.39393.
Zwart MF, Pulver SR, Truman JW, Fushiki A, Fetter, RD, Cardona A, Landgraf M. (2016). Selective Inhibition Mediates the Sequential Recruitment of Motor Pools. Neuron 91(3):615-628. DOI: 10.1016/j.neuron.2016.06.031.
Couton L, Mauss AS, Yunusov T, Diegelmann S, Evers JF, Landgraf M (2015). Development of connectivity in a motoneuronal network in Drosophila larvae. Curr Biol 25: 568–576, 2015. DOI: 10.1016/j.cub.2014.12.056. (see also Dispatch by Sternberg JR, Wyart C. Neuronal wiring: linking dendrite placement to synapse formation. Curr Biol 25: R190–1, 2015.)
Full list of publications via PubMed
