- A model synapse to study mechanisms of critical period adjustments
- Calcium dependent mechanisms to establish neuronal homeostatic setpoints
- Signals of change and mechanisms of maintenance
- Network connectivity is shaped by critical periods of development
- Computational modelling approaches to study the emergence of network function
A model synapse to study mechanisms of critical period adjustments
The Drosophila larval neuromuscular junction is a highly tractable model glutamatergic synaptic terminal. It has been key to our understanding of neuronal development, plasticity and homeostasis. We discovered that distinct critical periods, one after the other, govern the growth and operation of the pre-synaptic motoneuron and its post-synaptic target muscle.
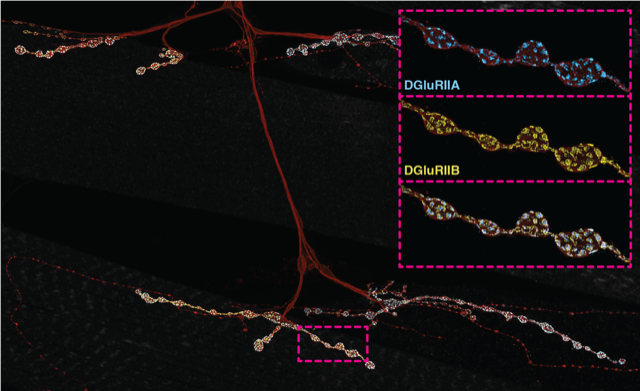
For example, in the muscle, this regulates sensitivity to neurotransmitter via relative levels of two glutamate receptor constellations, containing either a fast- or slow inactivating subunits.
Our working hypothesis is that by adjusting neuronal and muscle properties, the critical period establishes an appropriate physiological baseline about which plasticity can operate during later life.
Figure 1: Neuromuscular junctions as model synapses that readily reveal cellular adjustment responses to diverse critical period experiences. Motor axons (brown) arborise over muscles (grey), as beads-on-a-string synaptic terminals. Muscles localise neurotransmitter receptors to those; relative ‘A’ vs ‘B’ composition determines sensitivity to transmitter (inset).
Calcium dependent mechanisms to establish neuronal homeostatic setpoints
How do neurons translate transient activity-changes during the critical period, into long lasting adaptations of neuronal properties? Calcium is a good candidate signal, because it serves a dual function in neurons: as charge carrier and second messenger. To study the contribution of calcium signalling in regulating adaptive processes, we integrate electrophysiological and imaging techniques. A notable strength of this system is that we can manipulate single identified muscles and neurons, while leaving the rest of the animal undisturbed.
We aim to answer three interlinked questions.
- Is there a principal instructive calcium signal during the critical period?
- Where does this signal originate from?
- What are the downstream targets?
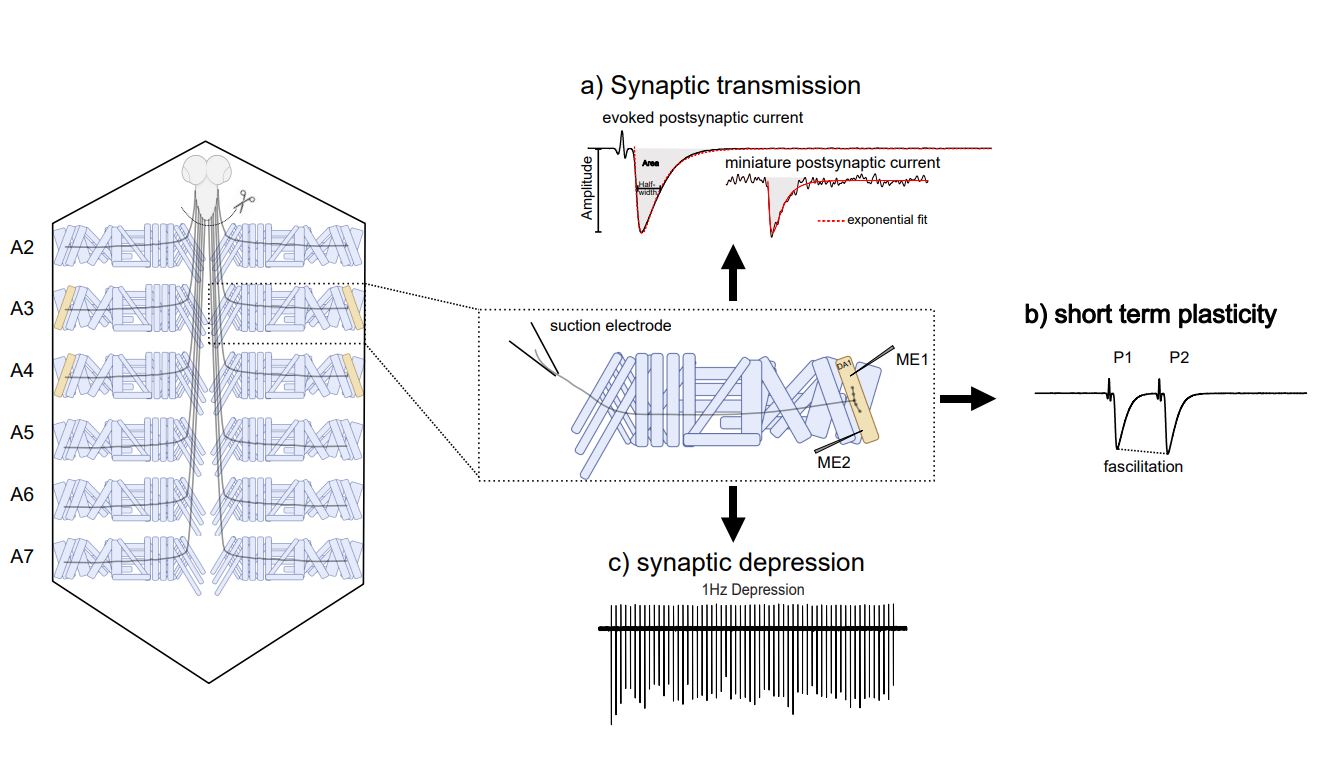
Figure 2: Two Electrode Voltage Clamp to analyse synapse physiology. The TEVC technique allows to target specific muscles and analyse postsynaptic currents, either spontaneous or induced, and analyse, depending on the stimulation paradigm, various synaptic properties, such as quantal content, short term plasticity or vesicle recycling.
Signals of change and mechanisms of maintenance
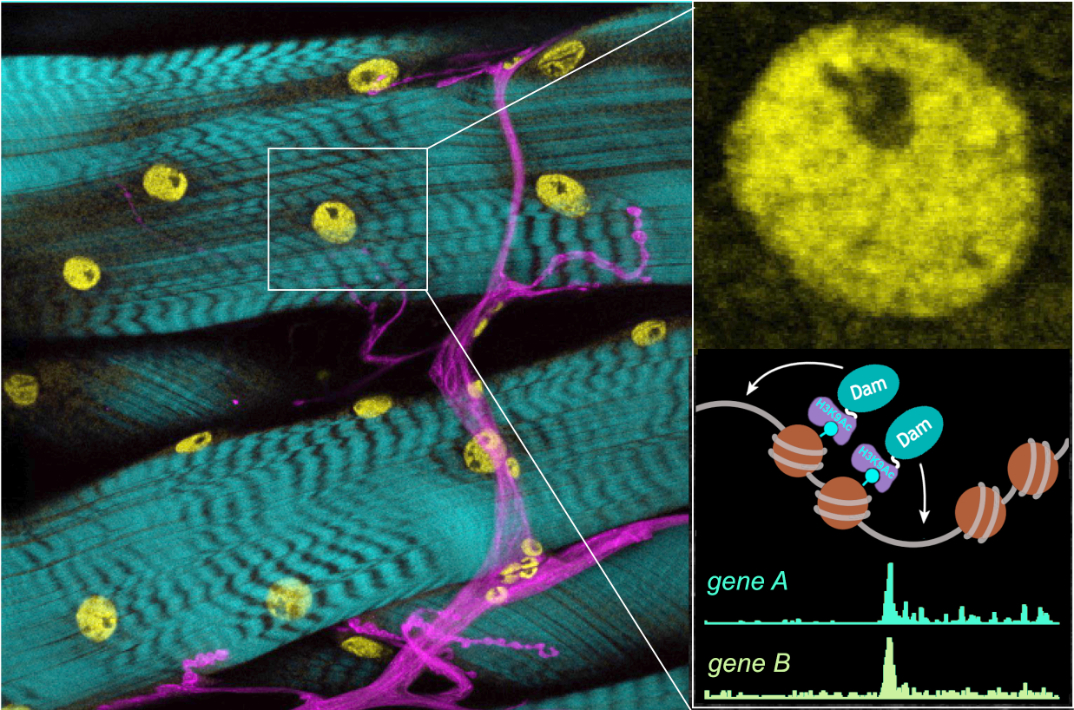
To identify the signals that instruct change during the critical period, we successfully adopted a best candidate approach and targeted genetic screens. We are now seeking a more detailed mechanistic understanding of how these signals operate. Related, we are working with collaborators, Jelle van den Ameele (MRC-Mitochondrial Biology Unit) and Tony Southall (Imperial College, London), to ask how such transient signals convert to lasting changes in gene expression and cellular properties. Here, we think that changes in epigenetic chromatic modifications are key, investigated using chromatin-DamID.
Figure 3: Epigenetic marks in muscle nuclei (yellow) change following different embryonic critical period experiences (nerves in magenta; actin in cyan). Enlarged inset shows muscle nucleus and chromatin DamID analysis strategy.
Network connectivity is shaped by critical periods of development
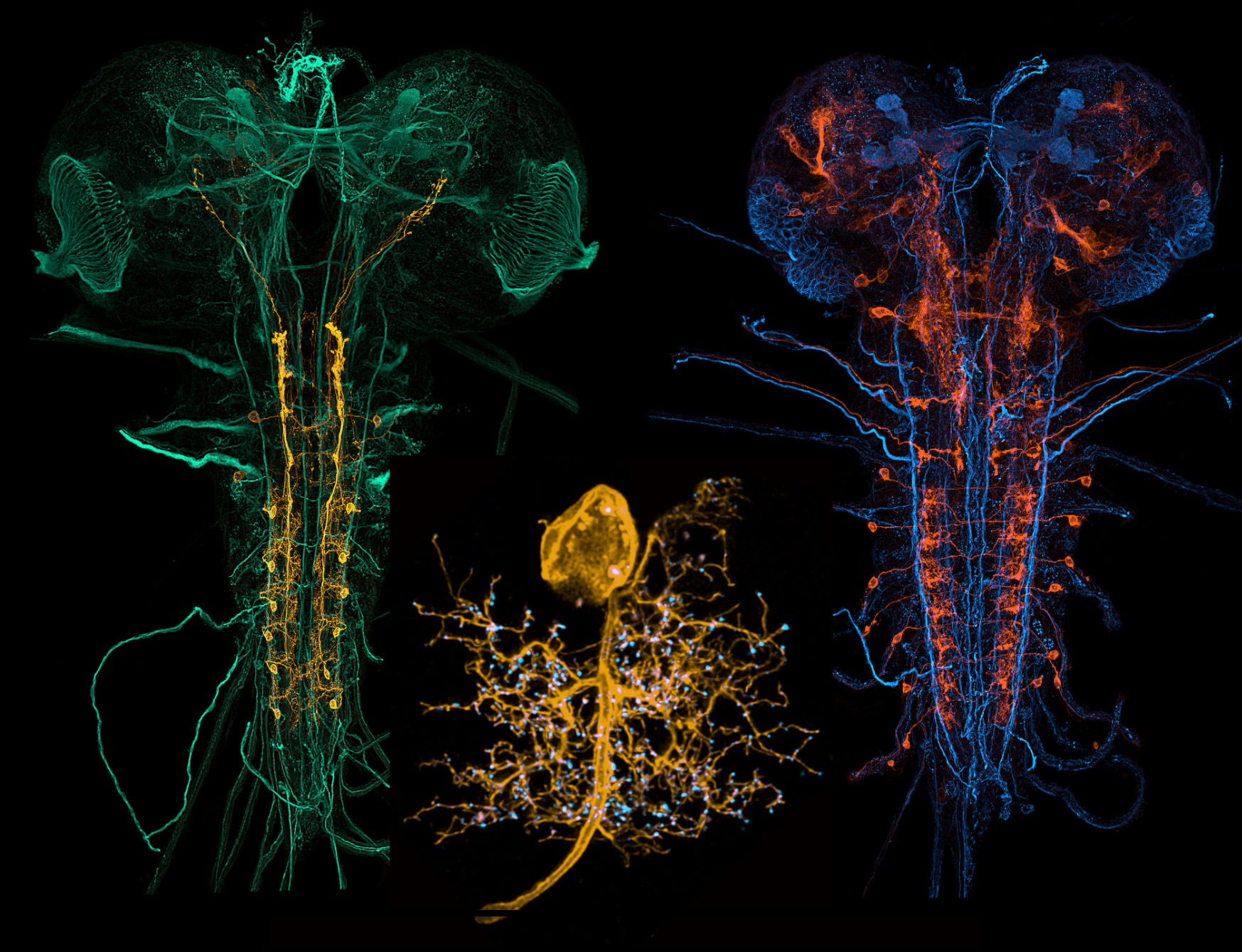
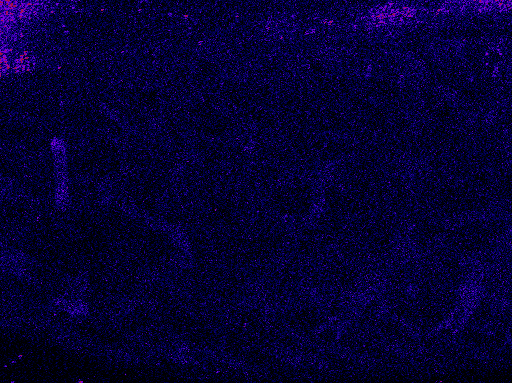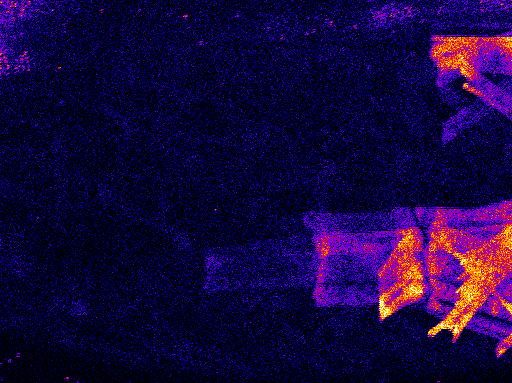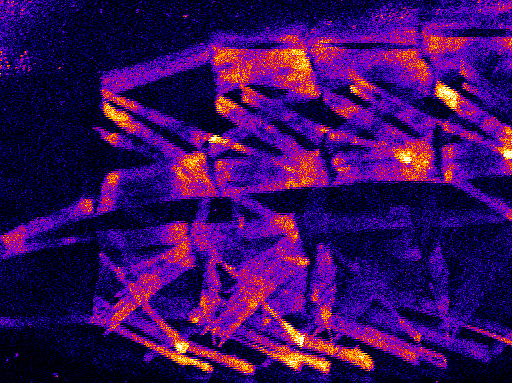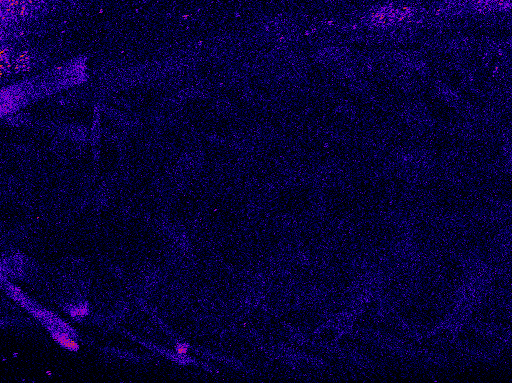
In the central nervous system, transient embryonic experiences during the critical period determine nerve cell growth and connectivity. We visualise these adaptations by high resolution imaging of fluorescent protein markers. Imaging of structural changes is complemented by electrophysiological approaches, conducted here and in collaboration with the Richard Baines group at the University of Manchester. This combined approach allows us to understand how nervous systems compensate for disturbances during development, so as to robustly generate appropriate function and behaviour.
Figure 4: Expression patterns of specific neuron types within the central nervous system of the Drosophila larva (left and right). Detailed view of a specific motoneuron (gold) and the pattern of synaptic input sites (blue).
Computational modelling approaches to study the emergence of network function
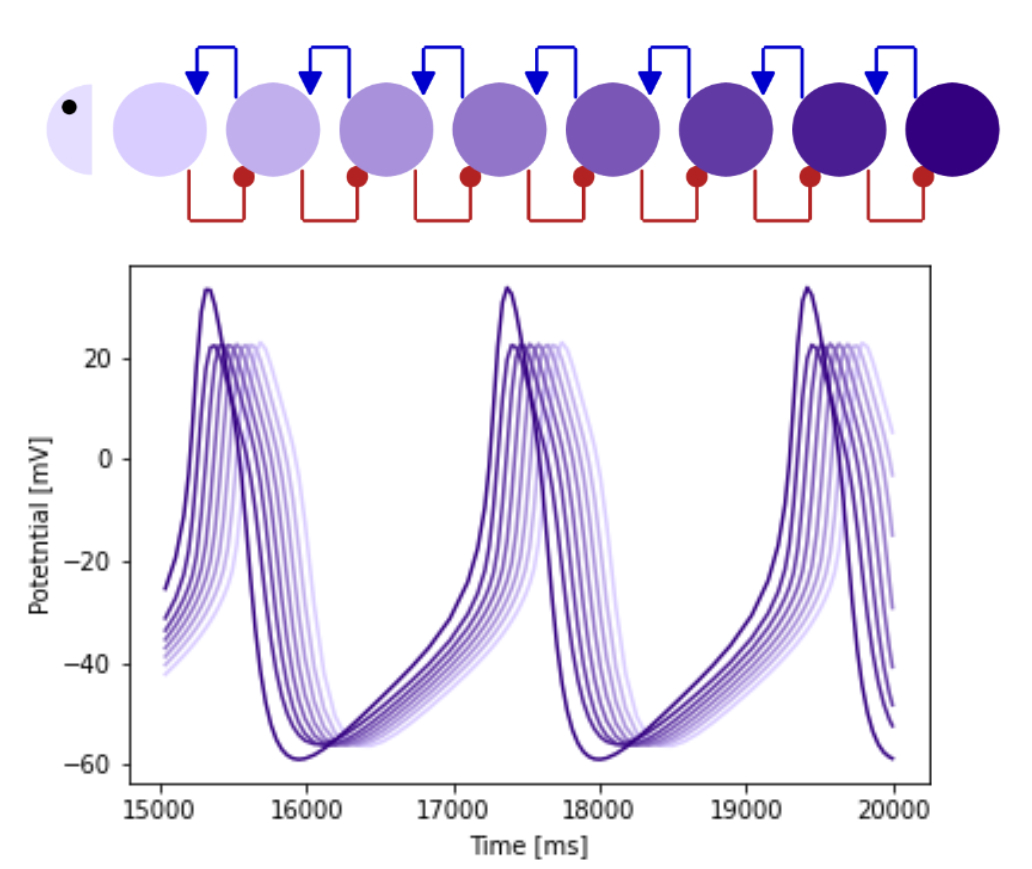
Using computational modelling we are investigating how changes in critical period network tuning affects locomotor output. Specifically, we study how changes in cellular excitability, connectivity, and synaptic transmission that result from critical period perturbations lead to changes in crawling behaviour. This work is enabled by a close collaboration with Timothy O’Leary (Department of Engineering). Asking what makes this network work well, during normal development, we have found that sensory feedback plays a crucial role in the control of this system. We are now focusing on the role of proprioception during the development of this network.
Figure 5: Patterns of locomotor network output, visualised by functional (GCaMP) imaging of the body wall muscles as the larva crawls (left). Modelling network adjustment during critical periods of nervous system development (right).