Setting the setpoint
Supervisor
Our brains are generally stable and do not go into seizures because the circuits (and also the neurons therein) use homeostatic adjustment mechanisms that work to maintain stable levels of activity. Central to homeostatic adjustment is the existence of a ‘setpoint range’. How this manifests itself is not clear – e.g. in terms of excitable properties, synaptic sensitivity, transmission, terminal size, etc.
Together with our collaborators (Prof. RA Baines, University of Manchester) we identified a defined developmental window in late embryogenesis, called ‘critical period’ when cells take stock of their environment and adopt homeostatic setpoints to match. We can now use advanced genetics to selectively manipulate the experiences of single, identified neurons and muscles during that period. This has already revealed some unexpected responses, for with we can now identify the underlying cellular mechanisms. Transcriptomic readouts will be used to identify the signalling pathways responsible. We expect many of those to link with human neuro-developmental conditions, which are increasingly thought responsible for psychiatric conditions that emerge during the adolescent critical period.
In this project you will use a combination of genetics, opto-and thermo-genetics and electrophysiology (2-electrode voltage clamp) to characterise the developmental events around the critical period. The readily accessible neuromuscular junction offers a great entry point, complemented by studies in the central nervous system. Through super-resolution imaging methods, such as expansion microscopy and STED we gain the necessary resolution to quantify structural changes at synapses.
References
1. Giachello CNG, Hunter I, Pettini T, Coulson B, Knüfer A, Cachero S, Winding M, Arzan Zarin A, Kohsaka H, Fan YN, Nose A, Landgraf M, Baines RA (2022). Electrophysiological validation of monosynaptic connectivity between premotor interneurons and the aCC motoneuron in the Drosophila larval CNS. J Neurosci. 42(35):6724–38. doi: 10.1523/JNEUROSCI.2463-21.2022.
2. Oswald MCW, Brooks PS, Zwart MF, Mukherjee A, West RJH, Giachello, CNG, Morarach K, Baines RA, Sweeney ST and Landgraf M. (2018). Reactive Oxygen Species Regulate Activity-Dependent Neuronal Structural Plasticity. eLife, 7. http://doi.org/10.7554/eLife.39393
3. Giachello CNG, Fan YN, Landgraf M, Baines RA (2021). Nitric oxide mediates activity-dependent change to synaptic excitation during a critical period in Drosophila. Sci Rep. 2021 Oct 13;11(1):20286. doi: 10.1038/s41598-021-99868-8.
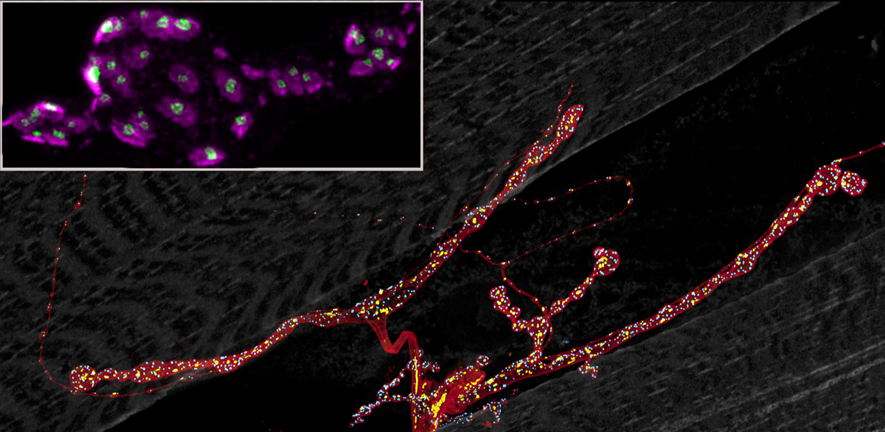
Image: Neuromuscular junction in the Drosophila larva. Motorneuron terminal size (brown), number of synaptic release sites (blue) and of mitochondria (yellow) are all regulated as part of its setpoint specification. Inset shows STED super-resolution image of postsynaptic receptor fields (magenta) juxtaposed to presynaptic release sites (green).
