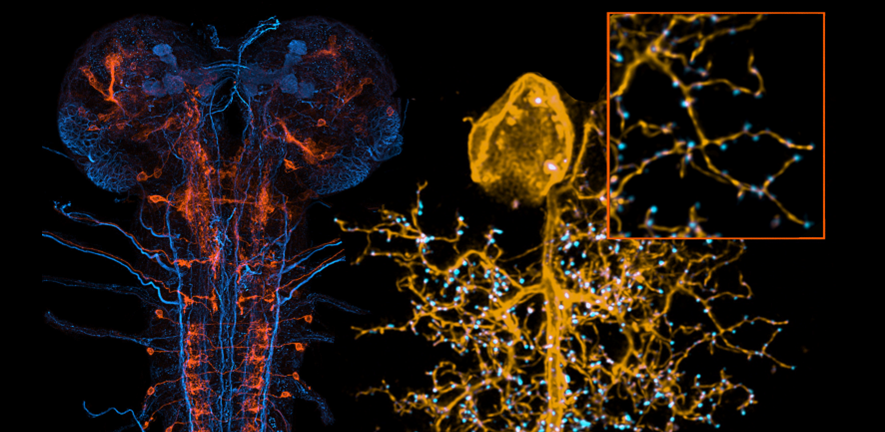
Image: Left: pre-motor interneuron (orange) in context of axon tracts (blue). Right: motorneuron (yellow) with postsynaptic sites (cyan); inset showing magnified view of dendrites.
Developmental robustness of neuronal networks
Supervisor: Prof Matthias Landgraf
Most animals are not warm-blooded and therefore have to buffer their development against biophysical impacts, e.g. seasonal temperature changes. Developing nervous system are particularly vulnerable to environmental impact: different temperatures experiences during a brief critical period (in late embryogenesis) permanently alters neuronal properties. This affects behaviour and can make animals seizure-prone - a concern as we face climate change.
What are the developmental, cellular and molecular mechanisms that endow developing nervous systems with stability?
We have discovered that during critical periods of development, metabolic signals specify cellular properties. We found that different parts of the developing locomotor network have distinct critical periods, suggesting that sequential specification might endow reliability.
Working with a well characterised network, that of the Drosophila larva, we are uniquely able to explore how critical period experiences change patterns of connectivity. We can ask how developmental timing directs network assembly. Or how we might re-open critical periods in later life to re-calibrate poorly adjusted networks.
References
Coulson B, Hunter I, Doran S, Parkin J, Landgraf M, Baines RA. (2022). Critical periods in Drosophila neural network development: Importance to network tuning and therapeutic potential. Front Physiol. 2022 Dec 2;13:1073307. doi: 10.3389/fphys.2022.1073307.
Giachello CNG, Hunter I, Pettini T, Coulson B, Knüfer A, Cachero S, Winding M, Arzan Zarin A, Kohsaka H, Fan YN, Nose A, Landgraf M, Baines RA (2022). Electrophysiological validation of monosynaptic connectivity between premotor interneurons and the aCC motoneuron in the Drosophila larval CNS. J Neurosci. 42(35):6724–38. doi: 10.1523/JNEUROSCI.2463-21.2022.
Giachello CNG, Fan YN, Landgraf M, Baines RA (2021). Nitric oxide mediates activity-dependent change to synaptic excitation during a critical period in Drosophila. Sci Rep. 2021 Oct 13;11(1):20286. doi: 10.1038/s41598-021-99868-8.
Sobrido-Cameán D, Oswald MCW, Bailey DMD, Mukherjee A, Landgraf M. (2023). Activity-regulated growth of motoneurons at the neuromuscular junction is mediated by NADPH oxidases. Front Cell Neurosci. 16:1106593. doi: 10.3389/fncel.2022.1106593.
