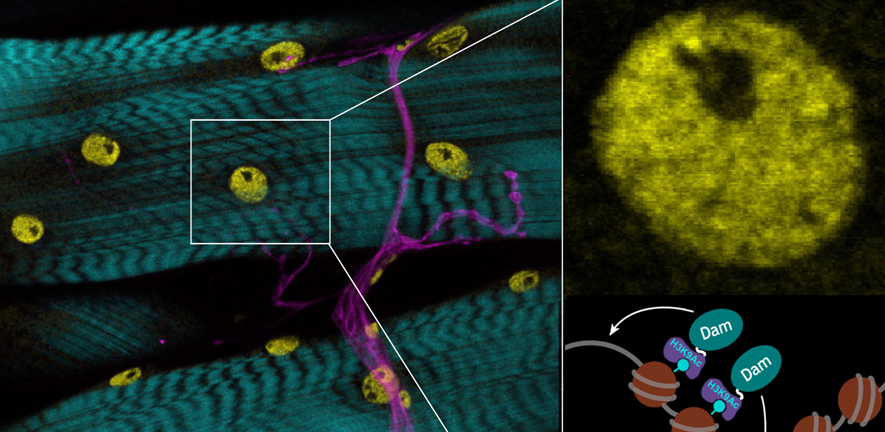
Image: Epigenetic marks in muscle nuclei (yellow), showing nerves (magenta) and actin (cyan). Enlarged inset shows muscle nucleus and chromatin DamID analysis strategy.
Epigenetic mechanisms during critical periods of development
Supervisor: Prof Matthias Landgraf
Co-supervisor: Jelle van den Ameele
Neuronal development requires the specification of functional properties, notably homeostatic setpoints; to allow for appropriate network function and stability. How this is achieved is poorly understood. We identified defined developmental windows (critical periods) during which this occurs; and metabolic reactive oxygen species as instructive signals. In humans, suboptimal critical periods and ROS dysregulation are thought responsible for neuro-developmental psychiatric conditions.
We are now investigating mechanisms by which transient disturbances during critical periods cause lasting change; in excitability, synaptic growth and transmission, which alter network function and behaviour. Working with Drosophila melanogaster as a model, we discovered that epigenetic modifications are linked to critical period manipulations. Now we can investigate how metabolic signals cause changes in chromatin marks, and how those are maintained through later life, effecting lasting change in cellular properties and function.
In this project you will use novel genetic tools for cell type-specific chromatin and transcriptomic profiling. You will use a range of techniques to characterise how critical period-regulated changes in gene expression lead to changes in neuronal structure and function. These include advanced genetics, opto-genetics, functional imaging, electrophysiology and behavioural paradigms.
References
Sobrido-Cameán D, Oswald MCW, Bailey DMD, Mukherjee A, Landgraf M. (2023). Activity-regulated growth of motoneurons at the neuromuscular junction is mediated by NADPH oxidases. Front Cell Neurosci. 16:1106593. doi: 10.3389/fncel.2022.1106593.
Coulson B, Hunter I, Doran S, Parkin J, Landgraf M, Baines RA. (2022). Critical periods in Drosophila neural network development: Importance to network tuning and therapeutic potential. Front Physiol. 2022 Dec 2;13:1073307. doi: 10.3389/fphys.2022.1073307.
van den Ameele J, Krautz R, Cheetham SW, Donovan APA, Llorà-Batlle O, Yakob R, Brand AH (2022). Reduced chromatin accessibility correlates with resistance to Notch activation. Nat Commun. 13(1):2210. doi: 10.1038/s41467-022-29834-z.
Oswald MCW, Brooks PS, Zwart MF, Mukherjee A, West RJH, Giachello, CNG, Morarach K, Baines RA, Sweeney ST and Landgraf M. (2018). Reactive Oxygen Species Regulate Activity-Dependent Neuronal Structural Plasticity. eLife, 7. http://doi.org/10.7554/eLife.39393
